Our planet is home to all variations of life and aging and survivors with unimagined abilities. The dayfly Oligoneuriella rehnana lives in the hatched stage for about 40 minutes, in which it mates in haste. Axolotl, the Mexican tail-worm, is able to regenerate limbs, organs and even parts of its brain, yet only reaches an age of about 20 years. The average life expectancy of a woman is 74.9 years, of which she is fertile only 30 years. The Greenland shark lives much longer with a life expectancy of around 400 years. The sequoia tree hangs here again a zero and becomes up to 4000 years old. The risk of dying remains the same for the freshwater polyps Hydra throughout their lives. These amazing caterpillars not only have a life expectancy of several centuries, but also the ability to regenerate if you cut the animal into five parts. A touch of immortality is also offered by the naked bull, which grows old without aging. With a life expectancy of over 30 years, it is an exception among rodents and also possesses amazing regenerative abilities.

Diversity of aging
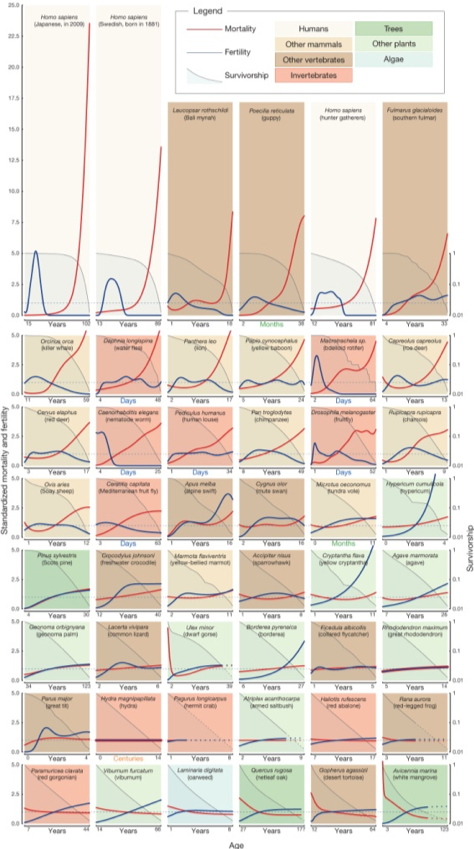
Life, reproduction and death are inextricably linked. It represents the eternal cycle of life and is the only thing that is actually immortal. Comparing the mortality, fertility and survival rates of different species reveals an extraordinary variety of survival strategies. They seem to have only one thing in common: At some point, everyone dies. There is still a long way to go from a general theory of aging. Even the seemingly logical assumption that the probability of dying generally increases with age is not always true. This is true for humans and also for primates such as chimpanzees. For some species, the probability of dying is the same throughout their lives. These include the above-mentioned examples of the hydra, the naked mullet, but also some sea anemones, some worms and the hermit crab. Hydra escapes decay thanks to its stem cells, which are present in greater numbers, as specialized body cells and a source of constant supply for new cells. In fact, it experiences almost exclusively extrinsic mortality. Nude mules have an extraordinarily efficient gene-reparation system, a very high protein stability and disrupt other signaling pathways that play a role in cancer development. Furthermore, since they do not have substance P and a differently constructed pain receptor, their pain perception is significantly reduced. Overall, it gives them a constant probability of death and a much longer life expectancy than that of other rodents. Other species can be observed to have a decreasing probability of death over the course of their lives: the color-changing coral gorgonie, the California gophers turtle or the oak Quercus Rugosa. This may be due to the fact that they are getting bigger and better able to withstand attacks from predators. In many birds, but also fish species, the probability of death increases strongly and then stabilizes on a straight line. The fertility of the species also knows all variations. The human woman is fertile for about 30 years of her life and continues to live for decades after the end of her fertility. The steppe-pavian and the alpine-sailer are fertile until their death. Greenland sharks only reach sexual maturity after 150 years of life, while the Pacific giant octopus, male ants and the male representatives of a pouquito species die immediately after reproduction. Also, the connection that more advanced species experience longevity cannot be established in general, since octopuses have a high intelligence and a short lifespan. The search for a unified theory of aging is difficult, but how do we age in the first place?
Nature 2013, DOI: 10.1038/nature12789 Published online 08 December 2013
How we age
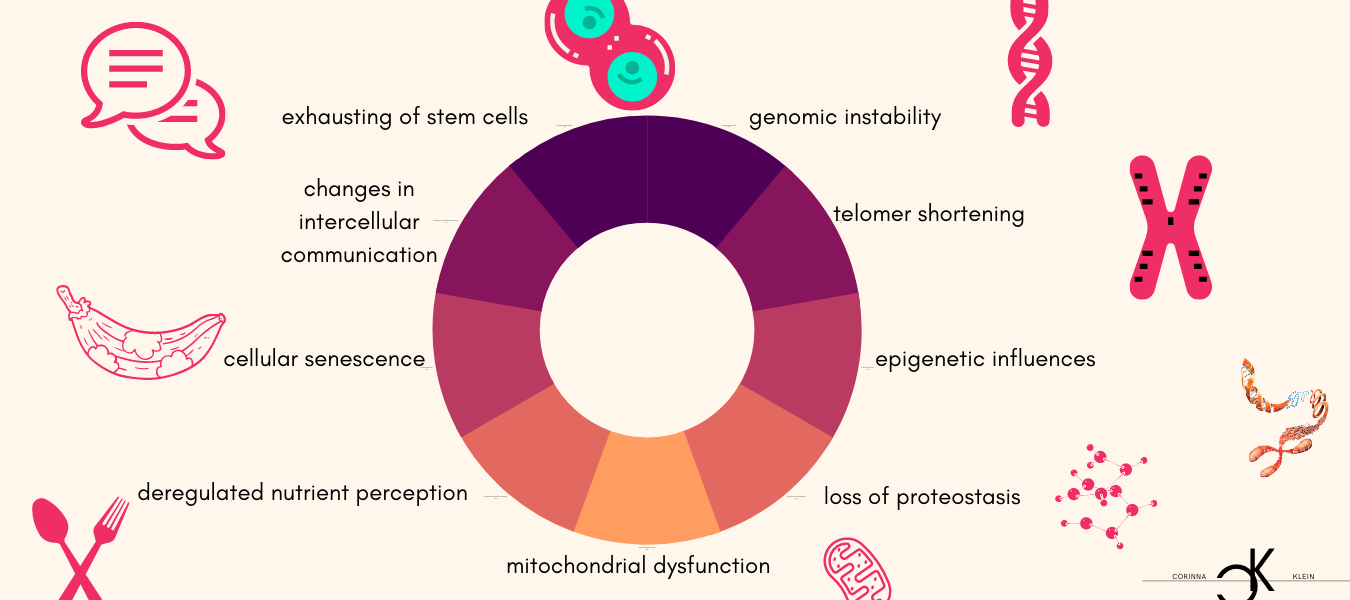
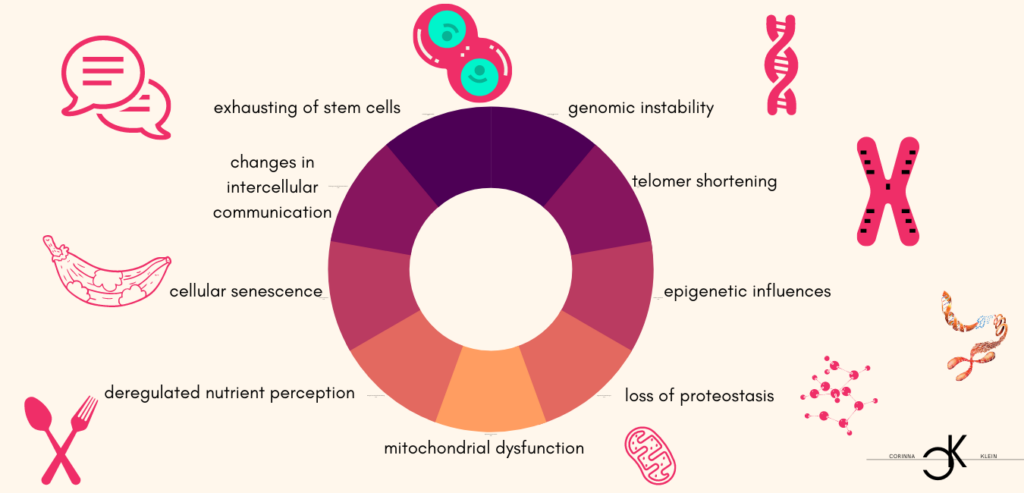
While it is largely clear which mechanisms at the molecular level are responsible for the aging process, the question of why is not yet unambiguous. As age progresses, damage accumulates that can no longer be repaired and leads to functional dysfunctions with an increased susceptibility to death. Essentially, nine factors are considered to be characteristic of aging in the different organisms: genomic instability, telomer shortening, epigenetic influences, loss of proteostasis, mitochondrial dysfunction, deregulated nutrient perception, cellular senescence, changes in intercellular communication and exhaustion of stem cells. The aging process is complex and consists of many molecular processes that are partly independent and partly based on one another.
Who wants to know more about it:
- Genomic instability: Our genome includes a blueprint for all necessary cellular processes and is contained as DNA (or RNA) in almost all cells. The genome is permanently exposed to harmful intrinsic and extrinsic influences (exogenous physical/chemical/biological agents, replication errors, hydrolysis, etc.). It is estimated that the genome of each cell is damaged up to 1 million times a day. Although there are sophisticated and highly effective self-repair processes, genome damage (point mutations, translocations, chromosome and telomeric changes, viral lesions, etc.) accumulate over time. Damage to the genetic material also favors the development of cancer. Certain diseases or lifestyle habits (smoking, being overweight, sunbathing) can speed up the aging process.
- Telomeric shortening: Telomeres represent the ends of the chromosomes and a shortening of these leads to genomic instability. One can imagine a telomer, like the hair gum at the end of a braided braid, without which the braid is unstable and disintegrates. Just as a hair gum becomes increasingly inelastic with each use and eventually breaks, the ends of the telomeres are worn off with each cell division. An enzyme (telomerase) is active to protect the telomeres, and its activity decreases with age until it is finally inactive.
- Epigenetic influences: The basis of life is formed by four letters that encode everything that nature produces in its infinite diversity. These four letters (A,T,C and G) encode a two-meter-long strand of DNA, which is wrapped and rolled up to fit into the tiny nucleus of the cell. The proteins around which the DNA in the nucleus is wrapped are called histones. Both may contain minor changes, such as methylation, which enable individual genes to be activated or deactivated. These changes form the epigenome, which also plays a role in aging, as the control over gene activity changes, among other things.
- Loss of proteostasis: Our genome encodes the blueprint of all proteins. However, in order for it to fulfil its function and function, it must be folded in a very specific way. Proteostasis describes the maintenance of the shape and quantity of all proteins. If a protein is folded incorrectly, it is ineffective or even toxic. The best known example of this is Alzheimer’s disease, which is caused by misfolded proteins. Although there are various control mechanisms and also a kind of cell-based waste collection, misfolded proteins increase with age and cause age-related diseases and dysfunctions.
- Dysfunction of the mitochondria : Mitochondria represent the cellular power plant that provides the energy needed for processes and chemical reactions. In them, the essential process of energy production from inhaled oxygen takes place. However, free radicals are also formed, which can have a harmful effect on intracellular molecules as well as an important role in the transmission of cellular stress, which affects, among other things, maintenance and repair processes. Mitochondrial dysfunction thus influences important singal pathways, processes and the performance of the cell and causes age-related diseases such as myo- and neuropathies.
- Deregulated nutrient perception: Cells respond to a sufficient supply of nutrients by storing energy and growing. However, when cells are exposed to a constant supply of nutrients, cellular mechanisms and their response to the supply become increasingly insensitive. This happens with obesity and diabetes, but also with increasing age. A number of studies have confirmed that the restriction of energy (reduced food intake) increases life expectancy. The nutrient sensor networks of the insulin/IGF (Insulin Growth Factor) and the TOR (Target of Rapamycin) metabolic pathway have proven to be particularly important indicators of aging. Inhibition of these networks prolonged life expectancy in animal experiments.
- Cellular senescence: Cells divide continuously to replace other cells or to allow the organism to grow. Most cells are unable to divide infinitely. At some point the capacity is exhausted and the cell passes into a supernatural, in which it does not die, but no longer divides. It becomes a senescent cell. One reason (of many) why cells become senescent is the shortening of telomeres or damage to DNA. The role of senescent cells is only beginning to be understood. It is clear that some of them release harmful molecules and harm the organism.
- Changes in intercellular communication: Intact cell-to-cell communication is essential for the functioning of our complex organism. Cells use a wide variety of communication channels: physical or chemical (releasing hormones/molecules). With age, both the ability to send and receive signals become worse. This results in inflammatory reactions, immune system weakness and also an increased susceptibility to cancer.
- Stem cell exhaustion: Stem cells are cells that can differentiate into different cell types or tissues. They represent a (theoretically infinite) source of new cells. They are only allowed to divide when new tissue is needed and must differentiate into the type of cell it needs at the moment. With age, this important increase in regulation is lost, so that, on the one hand, the source of new cells gradually runs out and, on the other hand, uncontrolled division leads to the development of cancer.
The search for a unified evolutionary theory of aging
As we begin to understand the processes that lead to aging, we are largely in the dark in the search for a unified theory of why we age. From a purely evolutionary point of view it seems like a paradox. Why is there a mechanism that causes damage in all kinds of ways? Why did evolution create aging? Looking at the approaches of evolutionary biologists Peter Medawar and George Williams, it can be concluded that aging has no evolutionary benefits, but is merely a side effect. Peter Medawar’s theory of mutation accumulation states that the force of natural selection remains high until the first reproduction and then decreases. The organism ensures the functioning of all processes up to the first reproduction and then continuously decomposes. Thus, mutations and negative effects of aging, which accumulate late in life, do not harm. It is no longer selected against them (selection shadow). The antagonistic Pleiotropy of George Williams also goes in a similar direction. Harmful genes and effects of aging can develop and become established, as they only develop at the end of the reproductive phase and thus have no evolutionary influence. Natural selection cannot select against genes, even if they ultimately cause the death of the organism, if these effects occur only at the end or even after the reproductive phase. In summary, aging is a side effect and not a planned, evolutionary process. However, this theory does not explain why some species, such as humans, continue to live for decades after their fertility ends and enjoy good health for a long time. Ronald Lee proposes to limit these theories not only to reproduction, but to complement the rearing of the offspring. Thus, not only the reproduction, but also the rearing is crucial. Many theories focus on higher organisms, partly because extensive data on invertebrate species, algae, fungi and bacteria are lacking. Thus, there are many different theories to this day, but there is no uniform and generally accepted scientific answer.