Do you remember those warm summer nights when suddenly glowing dots dance in trees? Or have you ever seen mystically glowing waves on the beach? Deep-sea fish, jellyfish, algae and the oil mushroom – nature turns on the light. Bioluminescence is one of the most fascinating natural wonders on Earth. Why do living beings produce light and what exactly is happening there?
Bioluminescence describes the ability of living beings to produce light. It is distributed in nature in completely different ways. For example, there are bioluminescent bacteria and fungi, crustaceans, insects and centipedes, fish and centipedes (including jellyfish). In mammals or higher plants, on the other hand, it does not occur at all. It is striking that bioluminescence is found much more frequently underwater than on land. The deeper the natural habitat of the animals in the sea, the more species are able to illuminate the infinite blackness of the sea with their own light. Some species produce the light themselves and others get the help of bacteria (symbiosis) that can produce the light. For example, the deep-sea anglerfish enters into a symbiosis with bacteria, which it supplies with oxygen and the bacteria take over the light generation for it. This is also called secondary lighting. One of the best-known examples of primary light is probably the firefly. It generates its light independently and we would probably ignore him during the day, as he comes in a nondescript brown. So far so good, but why do some species glow and especially how?

Why do some species glow?
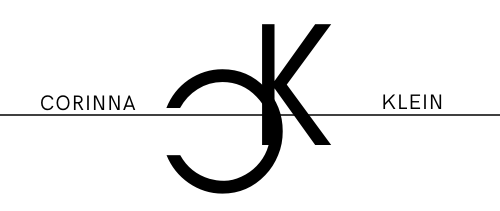
There are different ways in which the light is produced and it is found in completely different species of animals. It is thought to have evolved and become established in species independently. It does not always serve an identifiable purpose and, in the case of bacteria for example, may simply be an accidental by-product of a chemical reaction. In many species, however, bioluminescence is actively used for the following reasons:
- mating
- camouflage
- communication
- foraging
- as a deterrent, deception, warning or threat
The Chemistry Behind Lighting
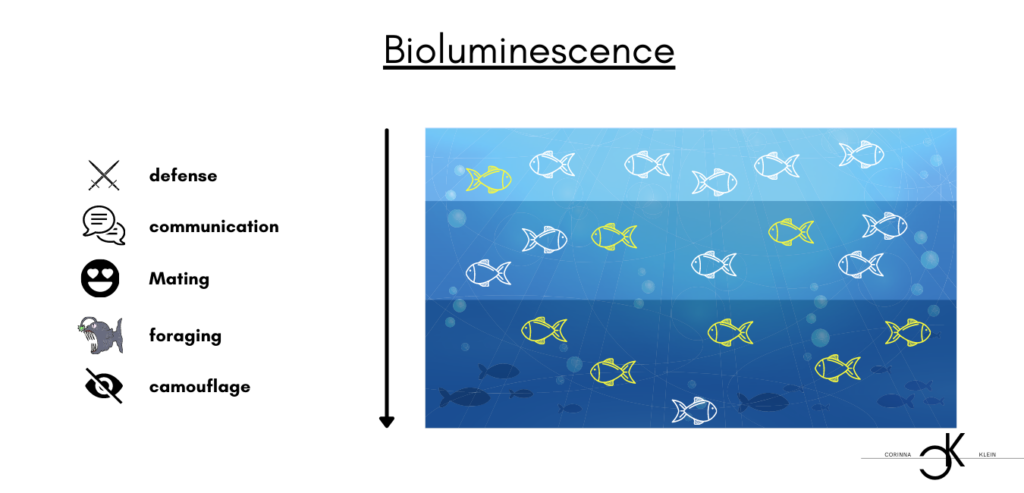
Bioluminescence is actually a chemiluminescence. The formation of light is based on a chemical reaction in which the energy is released in the form of light. Not only one color, but different colors are created. Essentially, these are blue, green and yellow. As mentioned above, bioluminescence has not only one origin, but has been “invented” several times independently of each other in the different species. As a result, there are different reactions in different types, and thus also differences in the course of the chemical reactions in which the light is produced. In most of these reactions, oxygen reacts with luciferins to produce light. Luciferins are a class of natural products used by living beings to produce bioluminescence. Enzymes are needed to catalyze the reaction. These are called luciferases and probably evolved from the oxygenases in the course of evolution. Let’s take a closer look at the chemistry behind it. In the presence of Ca 2+ ions, the enzyme luciferase catalyzes the exergonic oxidation of luciferins with molecular oxygen (O2) to dioxetanes or dioxetanone. The latter is unstable and decays in nanoseconds emitting carbon dioxide (CO2), releasing energy in the form of light. Let’s go through this again slowly: for the reaction to start, the calcium ions and luciferase are needed. As a catalyst, luciferase provides the necessary jump-start by lowering the activation energy to the point where the reaction starts. An oxidation is a chemical reaction in which the substance to be oxidized gives up electrons, which are taken up by another substance. In our case, Luciferin picks up more electrons. This process consumes oxygen. Exergonic reactions describe reactions that occur voluntarily and in which the free enthalpy decreases, i. e. the system moves towards a state of equilibrium. Dioxetane/ dioxetanone is a four-ring electronically excited and decaying compound. Photons are released during this decay, and it is precisely these photons, or light quanta, that are small packets of energy whose energy we see in the form of light.
A look into the future – what can we learn from nature?
The reaction described above, which ultimately produces light, is many times more efficient than the methods humans use to produce light. With a conventional light bulb, 90% of the energy is wasted on heat and only 10% of the energy becomes light. The luminous efficiency of bioluminescence is about 98%, only about 2% is converted into heat. For this reason, bioluminescence is a so-called cold light. If nature turns on the light so much more efficiently than we do, what can we learn from it?
Bioluminescence is already used as a marker method in molecular biology and as a detection of toxins in ecotoxicology. A team of researchers led by Karen Sarkisyan and Ilia Yampolsky described initial successes in the genetic modification of a tobacco plant that has a self-sufficient luminescence visible to the naked eye. Perhaps one day our street lamps will be replaced with luminescent trees? Too much light, however, would be harmful to the natural role models. Already now, the numbers of the fireflies are declining further and further, as the increasing lighting during the night makes it more difficult to find a mate. Maybe we should sit in the garden more often and let ourselves be fascinated by the glowing butts of these little energy machines in the dark.