Who doesn’t know this moment? At the end of a day, the sun crowns its sunset (which, in more detail, we actualy do the sunset and the sun stays where it is) with a phenomenally dramatic play of red, yellow and violet tones that make the heart of any romantic beat faster. But what do we actually immortalize millions of times in our photo albums and Facebook or Instagram accounts?
In general, the sky has almost the entire color palette to offer. A cloudless sky appears blue to us during the day, although behind it is the infinite blackness of space. At sunrises or sunsets red, yellow and violet are added. How does this happen, when it is always the same sun that produces the light? Sunlight is that part of the electromagnetic radiation emitted by the sun that we can see (visible light, about 780 – 380 nm). The different wavelengths correspond to different colors. The wavelength is shortest for blue light and longest for red light. Although the sun appears yellowish to us, visible light is made up of all the different wavelengths.

On the path of light from the sun to the earth, light encounters resistance and obstacles. In the atmosphere, for example, it collides with gas molecules such as oxygen or nitrogen, which cause the light to be deflected, i. e. scattered. Light scattering in the atmosphere by very small particles is Rayleigh scattering. One speaks of Rayleigh scattering whenever radiation is scattered by particles that are smaller than the wavelength of the radiation. As mentioned above, the energy increases with decreasing wavelength. Blue light, with the shortest wavelengt, is therefore the most energetic. Blue light is scattered four times more than red light, because the power with which the particles re-emit the absorbed radiation is inversely proportional to the fourth power of the wavelength of this radiation.
For those who want to know exactly:
Through the action of the light of a certain wavelength, a force is exerted on the charge of the molecule and its dipole, which thereby becomes vibrating. The vibration corresponds to the frequency of the charge. As a result, the molecule begins to periodically reverse its polarity and thus becomes a kind of mini-transmitter. The radiated power is proportional to the square of the dipole torque and to the fourth power of the vibration frequency. At small wavelengths much more radiation occurs, as the ratio of the dipole size to the wavelength is more favorable. As mentioned above, blue light is therefore four times more scattered than red light. This can also be illustrated by the following considerations. If you dip your hand into a bowl of water and move it quickly and evenly back and forth, many small waves are formed that spread in almost all directions. However, if you move your hand very slowly, there are longer waves that are hardly visible.
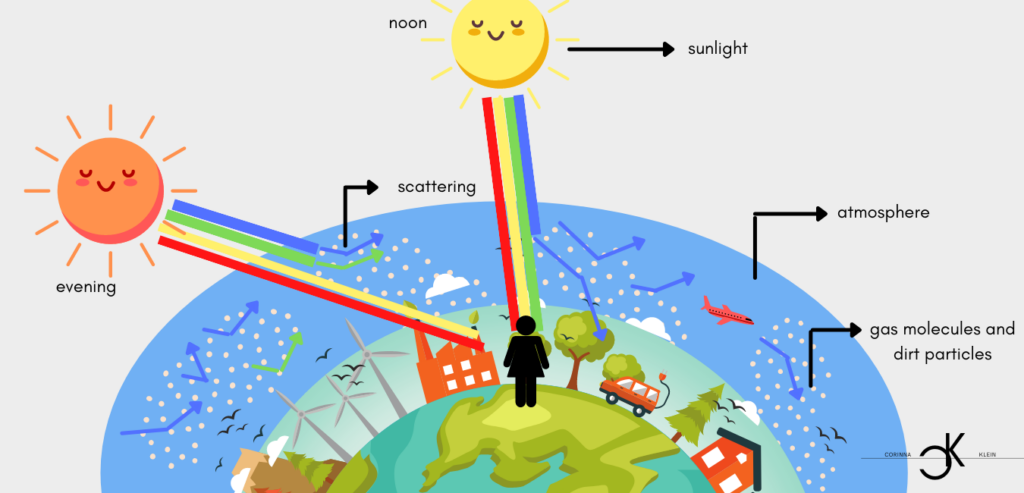
During the day, when the distance between the sun and the earth is smaller and less distance has to be covered, a lot of blue light is scattered. It is deflected in all directions. With this scattered radiation, the proportion of blue light clearly predominates and makes the sky look blue. The sun appears yellow to the viewer due to the lack of blue. Where in space, as for example on the moon, no dense atmosphere exists and thus hardly any Rayleigh scattering can take place, the sky also appears black during the day and the sun white (due to the superposition of the spectral colors).
In the morning or in the evening, however, the path of light through the atmosphere is much longer. This means that the light hits many more scattering particles. As a result, a large part of the blue light is scattered away, it is intercepted by the molecules, so to speak, and no longer reaches the eye of the observer. This leaves mainly light with long wavelengths. This makes the sun and the sky appear red, yellow or purple. This effect is further enhanced by various particles in the air (haze, aerosols and dust). Many of these particles are contaminants. One of the reasons for romantic sunsets is therefore nothing more than dirt particles scattering away short-wave light.